
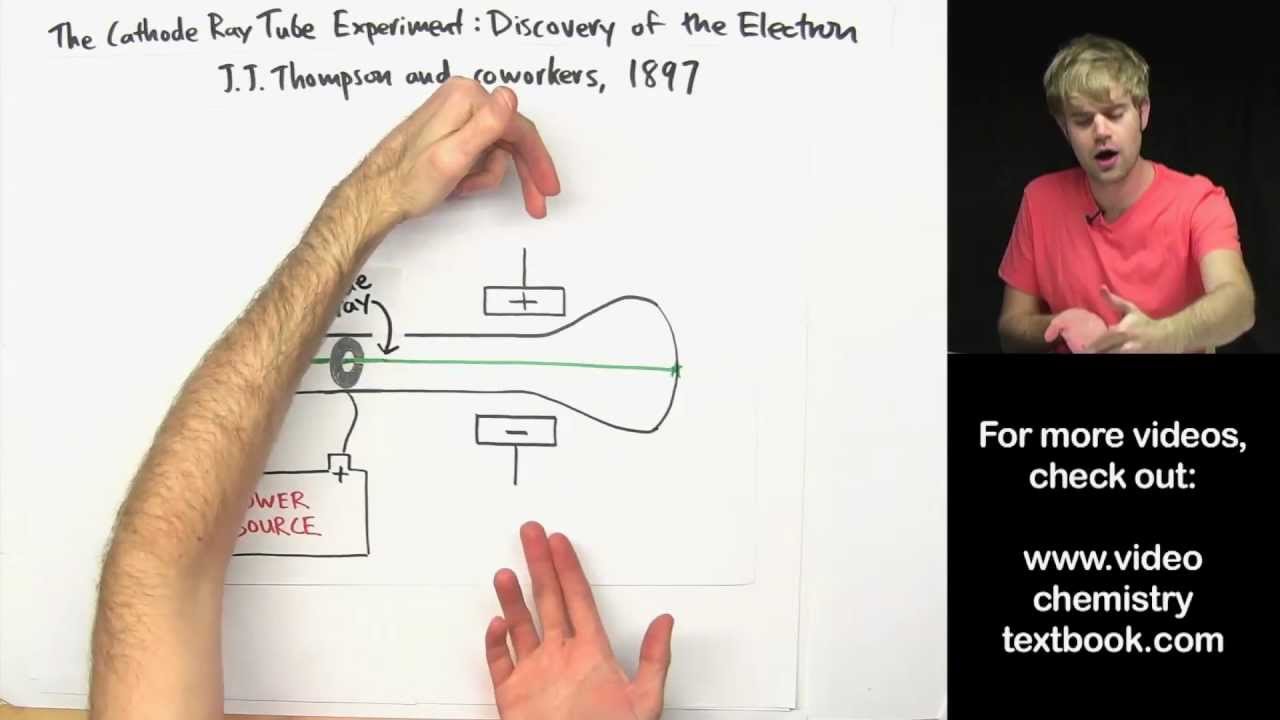
Democritus called this part as the atomos. Prior to Dalton’s atomic theory, the famous Greek philosopher, Democritus, discovered the very basic theory about atom. He created the theory from the concept of there should be the part of matter which is extremely small that we as a human can not see. There were multiples number ratios in various compounds like oxygen compound. Atoms may combine in more than one-all ratios in element reactions.

Based on Dalton’s experiment, he concluded that the chemical reactions will occur based on atom to atom ratios

Atoms combine in the small and whole-all rations in chemical reactions.While atoms in the different element will have the different characteristic from one to another. Every single atoms in oxygen is same to another. Dalton believed that all atoms in the same element must have same weights. The weight of atom determines the characteristic of atom.He used the Antoine Lavoisier theory to support this point The atoms in element can not be created, destroyed, divided or transformed. Atom can not be destructed and changed.He also said that atoms have various motions He belived that atoms has the small shape and solid spheres form. All mater contains very small particles which are called atoms.His theory has five basic principle as following points: John Dalton conducted the atomic theory called the five postulates of John Dalton. In 1803, John Dalton released his atomic theory which will then become one of the most useful theory in chemistry. Prior Study by Dalton that Triggered JJ Thomson Scientists Who Contributed to The Atomic Theory.


 0 kommentar(er)
0 kommentar(er)
